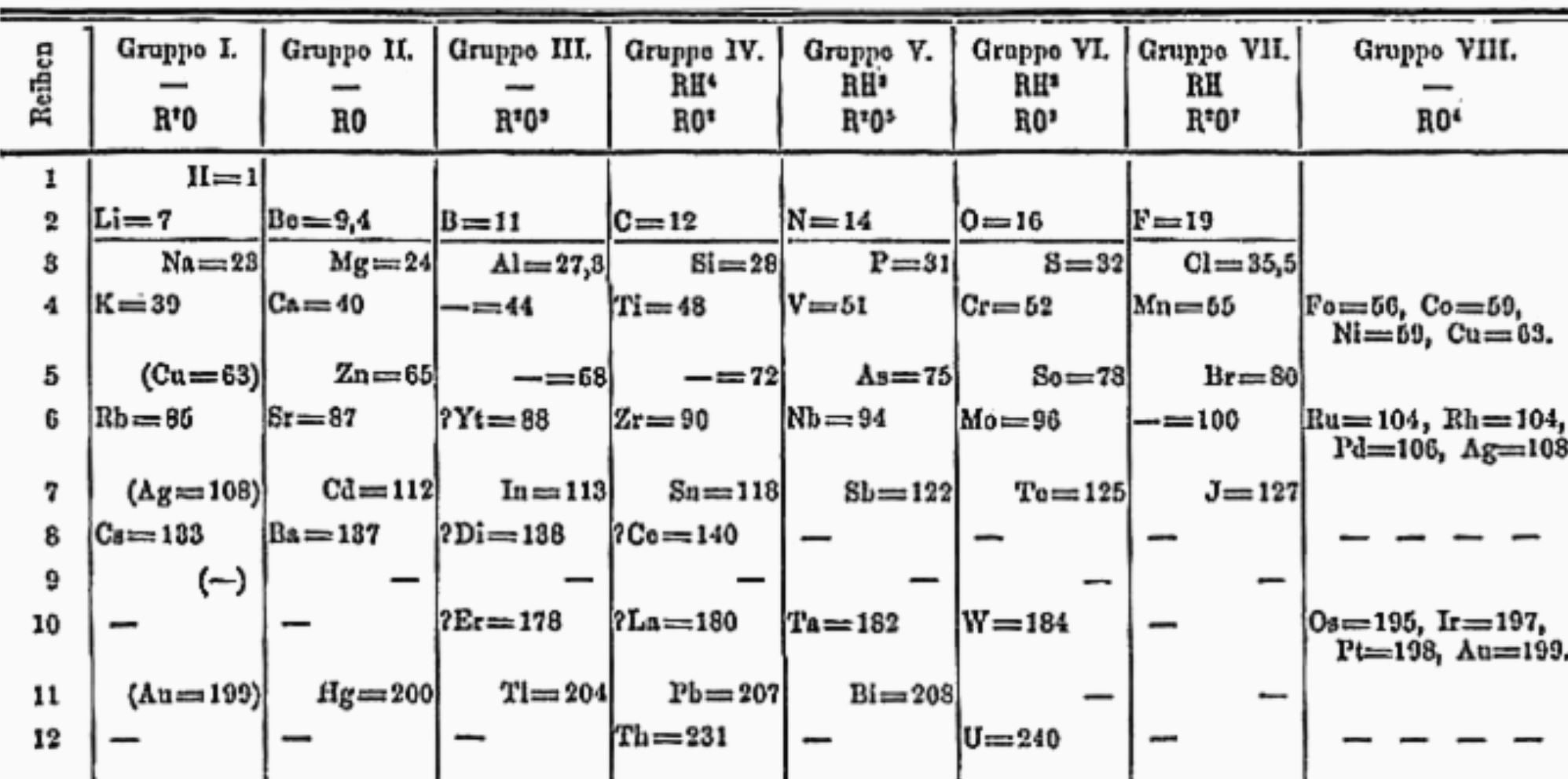
At that epoch more than 60 elements were known; today, the number has almost doubled (see Figure 2). The last 4 elements (nihonium, moscovium, tennessine e oganesson) were officially presented in 2016.
The Periodic Table includes both stable elements (i.e. with an endless lifetime) and unstable elements (i.e. they transform to another chemical species).
In nature, all elements can be found, apart from the Superheavy ones, created artificially and with extremely short lifetimes.
In the Periodic Table, elements are ordered
with increasing atomic numbers (symbol Z), i.e. with increasing number of “protons” within the nucleus. The proton is a sub-atomic particles, with a mass equal to 1.7x10-24 gr
(about 2000 billionths of billionths of billionths of gram) and a positive charge equal to 1.6x10-19 C (16 billionths of billionths of Coulomb, which is the charge transported in 1 second by an electric
flux of 1 Ampere). Atoms are globally neutral, because around nuclei (positively charged) there are electrons, with negative charge.
Inside nuclei there are also neutrons, which have a mass almost identical to protons, but without charge. Therefore, an atom consists of a nucleus (with protons and neutrons), surrounded by a "cloud" of
electrons. The nucleus charge provides its atomic number (Z); its mass, instead, is commonly identified by the sum of nucleons
(A=protons+neutrons: the masses of electrons are negligible with respect to nuclei).
Each chemical element has its typical "isotopic composition". “Isotopes” are atoms with the same atomic number, but with a different number of neutrons.
In nature, there are mono-isotopic elements (as fluorine or gold), elements with many stable isotopes
(tin has 10 isotopes: the largest number) and elements without stable isotopes (as technetium, which transforms to molybdenum).
The interested reader can explore the IUPAC Periodic Table of the Elements and Isotopes .
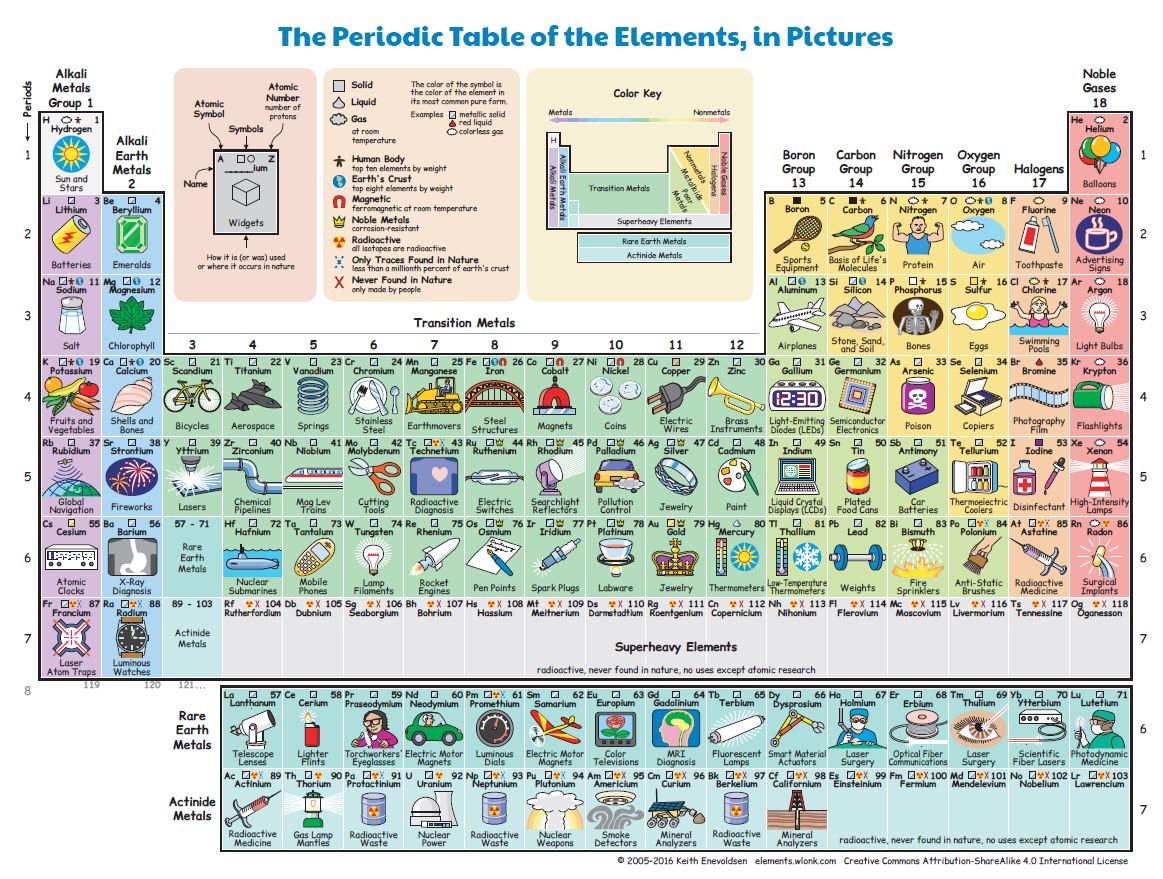
Download Figure 2 (pdf;jpg)