In the last 100 years, physicists and astrophysicists tried to answer the following question: where do the chemical elements come from?
The physical conditions on the Earth do not allow the production of new elements, because very high temperatures are needed (at least one million degrees). As a consequence, all the elements known were
already present in the proto-Earth at the epoch of its formation (which corresponds to the epoch of Sun formation, considering that our planet formed from the same material of our star).
Let's take our Sun as a reference to understand which are the most abundant elements in the Universe (in mass). In the Sun, the two lightest elements (hydrogen and helium)
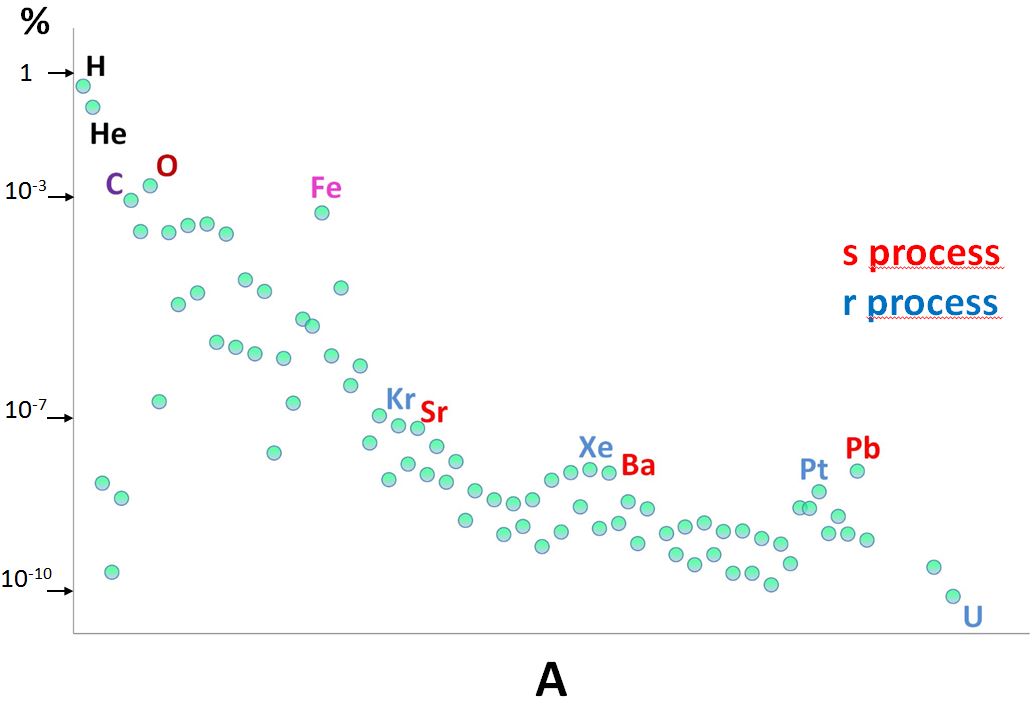
represent about 98% of its mass (H at 70%, He at 28%). Figure 3 shows the distribution of the solar surface abundances (if not directly measurable, other sources have been considered).
The scale of the vertical axis is logarithmic, necessary to properly visualize in a single plot abundances spanning about 10 orders of magnitude (from hydrogen to uranium, which is about 10 billion times less abundant).
Hydrogen and helium, as well as lithium, formed in the first hour of the Universe, while it was yet rapidly cooling after the Big Bang. Other elements formed in stars which evolved in the
following billions of years. The only exceptions are beryllium and boron, which have been mostly created by spallation induced by cosmic rays.
