2019: INTERNATIONAL YEAR OF THE PERIODIC TABLE
by Sergio Cristallo
The production of elements heavier than iron is definitely more complex, since there are no stable nuclei
with atomic mass A=5 and A=8. Therefore, proton capture processes are not a viable solution to synthesize
heavier species.
In 1951 Ernst Opik, and later in a more detailed way, Edwin Salpeter (Craaford prize in 1997),
hypothesized that in Giant stars the energy counterbalancing gravity comes from the
burning of 3 helium nuclei. In the Sun, this type of reactions cannot occur
because the solar core is not hot enough and, as a consequence, the Coulomb repulsion prevails
(i.e. two particles with the same charge push each other apart).
Helium burning occurs in two steps. During the first phase, two α particles (an acronym for helium
nuclei) merge to form a 8Be nucleus, which in turn captures another
α particle, producing 12C. The problem with this theory
is the short 8Be lifetime, which decays back into two α particles in about
2x10-16 seconds (that is in about 20 millionths of millionths of second). The solution to this
puzzle came in 1953 thanks to the English cosmologist Fred Hoyle (Craaford prize in 1997), who
postulated the existence of a resonant energy level in the atomic structure of 12C. This
state was later experimentally confirmed at Kellogg laboratories of the
California Institute of Technology, from the group led by William Fowler (Nobel prize 1983).
People refer to this level as the
“level of life”, because the existence of carbon made possible the development
of the life. The human biology, in fact, is based on the carbon cycle.
Once produced, however, 12C can capture another α particle, forming
16O. A new species appeared:
oxygen! It would be trivial to magnify the importance of oxygen for us (just let us cite the fact that without oxygen we would not have water, being its stoichiometric formula
H2O!!!). In the solar composition, oxygen is the most abundant element after hydrogen and helium (and carbon is on its heels; see Figure 3).
Actually, it's the most abundant element in the Earth crust (see Figure 5): this demonstrates how much different planetary compositions are with respect to the stellar ones (and if we look to another planet, we would
find further differences among planets).
Figure 5: COMPARISON BETWEEN CHEMICAL COMPOSITIONS
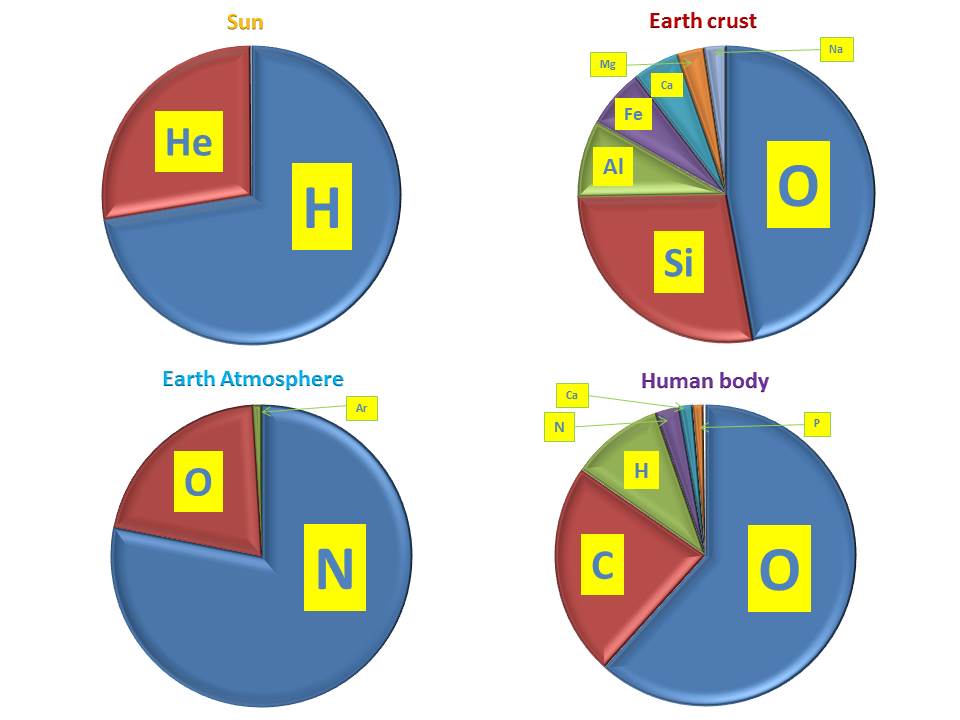
As it is shown in Figure 5, the crust composition strongly differs from the atmosphere. The latter, in fact, is dominated by nitrogen.
This element forms through the so-called “CNO cycle”: an alternative burning channel with respect to that occurring in Sun interiors.
This process, studied in detail by Hans Bethe (Nobel prize 1967), is efficiently activated in stars slightly more massive than the Sun.
We also highlight that the chemical composition of a human body is still different, being dominated by oxygen, carbon and hydrogen (all in all we do not need that many elements to survive, even if
"the devil is into details"!).
It's funny to realize that our bodies last for less a century, while the atoms constituting them
(some billions of billions of billions) are AT LEAST 5 billion years aged!!!
